FDA Approved First Vaccine for Pregnant Women to Prevent RSV in Infants Six Months Old and Younger
Though this is not a Pfizer or Moderna COVID vaccine report, it offers vital information on a Pfizer vaccine targeting a once protected group -- pregnant women and their unborn babies.
Remember just a few short years ago when Americans were told that pregnant women should take as few medications as possible?
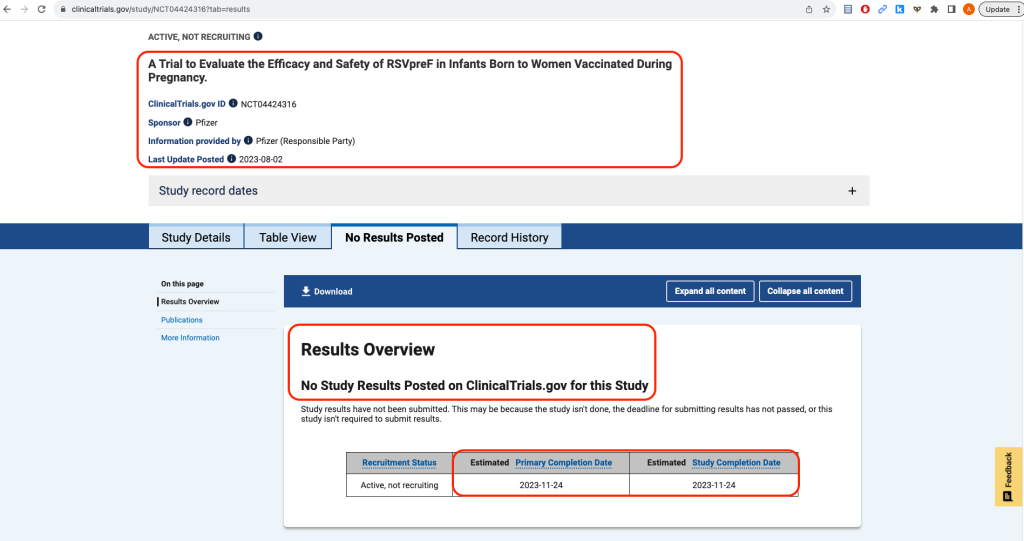
On August 21, 2023, the United States Food and Drug Administration (FDA) approved an RSV (Respiratory Syncytial Virus) vaccine, Pfizer's Abrysvo, for use in pregnant women at 32- to 36-weeks gestational age to allegedly protect their newborn babies, up to six months of age, from RSV. Pfizer notes, "The FDA’s decision is based on the data from the pivotal Phase 3 clinical trial (NCT04424316) MATISSE (MATernal Immunization Study for Safety and Efficacy), a randomized, double-blinded, placebo-controlled Phase 3 study designed to evaluate the efficacy, safety, and immunogenicity of the vaccine against LRTD and severe LRTD due to RSV in infants born to healthy individuals vaccinated during pregnancy. These results were published in The New England Journal of Medicine in April 2023." [https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-abrysvotm-pfizers-vaccine-prevention-0] The MATISSE study was funded by Pfizer. Clicking on the clinicaltrials.gov link -- "(Funded by Pfizer; MATISSE ClinicalTrials.gov number, NCT04424316.)" -- provided in the New England Journal of Medicine article, one finds that the study does not show as being complete and that no results have been posted:
Yet, the FDA has approved the use of this new drug in pregnant women, traditionally one of the most medically protected demographics.
The Pfizer press release goes on to state:
“Newborns and young infants – whose immune systems are still developing and are not yet strong enough to defend against infections – may now be protected from RSV from the moment of birth through maternal immunization,” said Eric A.F. Simões, M.D., Clinical Professor, Pediatrics-Infectious Diseases, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora. “The approval of Pfizer’s ABRYSVO is a major triumph as it helps ensure no delay in potential RSV protection during an infant’s most vulnerable first six months of life and offers healthcare providers a new opportunity to help prevent severe RSV.” [Emphasis added.] [https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-abrysvotm-pfizers-vaccine-prevention-0]
That language -- "may" and "potential" -- and the underwhelming findings, as seen below, fail to offer a convincing reason that their target demographics -- pregnant women and their unborn babies -- should be subjected to this novel drug.
A review of the results published in the New England Journal of Medicine shows:
"The percentages of maternal participants with any adverse events reported within 1 month after injection were similar in the vaccine group (13.8%) and the placebo group (13.1%)."
"The percentages of infant participants with any adverse events reported within 1 month after birth were 37.1% in the vaccine group and 34.5% in the placebo group."
"Among the infant participants, the incidences of adverse events of special interest and newly diagnosed chronic medical conditions - including Developmental Delay, Low Birth Weight, SARS-CoV-2 Test Positive, and Prematurity - were similar in the two groups."
"Among maternal participants, the incidences of serious adverse events through 6 months after injection were similar in the two groups; the most frequent were preeclampsia (in 1.8% of participants in the vaccine group and 1.4% of those in the placebo group) and fetal distress syndrome (in 1.8% and 1.6%, respectively)."
"The incidences of premature delivery were similar in the two groups (28 cases [0.8%] in the vaccine group and 23 cases [0.6%] in the placebo group)."
"Serious adverse events in four RSVpreF vaccine recipients (pain in an arm followed by bilateral lower-extremity pain, premature labor, systemic lupus erythematosus, and eclampsia — in one recipient each) and in one placebo recipient (premature placental separation) were assessed by the investigator as being related to the injection."
"The incidence of reported serious adverse events - including Newborn Transient Tachypnea, Respiratory Distress, Prematurity, Low Birth Weight, Neonatal Jaundice, Hypoglycemia, Neonatal Sepsis, Neonatal Hyperbilirubinemia, Atrial Septal Defect, and Ventricular Septal Defect - in infants from birth through 24 months was similar in the two groups."
And, of course: "No serious adverse events in infants were considered by the investigators to be related to the vaccine."
The Centers for Disease Control and Prevention (CDC) currently gives the following approximate annual statistics for each year for RSV-related outcomes for children younger than five years of age:
2.1 million outpatient (non-hospitalization) visits among children younger than 5 years old.
58,000-80,000 hospitalizations among children younger than 5 years old.
100–300 deaths in children younger than 5 years old.
In 2023, the United States saw 3,745,361 babies born. The high end number, 300, of the CDC-published number of yearly RSV deaths in children under five-years-old equates to a potential 0.0080% fatality rate. The calculation is not apples to apples since the numerator represents babies and the denominator represents all children under age five. That means that the fatality rate is likely much lower than 0.0080% for infants. Given that microscopic fatality rate, we should consider the possible adverse events associated with the Abrysvo vaccine, which has not been fully studied, according to the CDC:
In the clinical trials, the side effects most often reported by pregnant people who received the maternal RSV vaccine were pain at the injection site, headache, muscle pain, and nausea.
"...a dangerous high blood pressure condition called pre-eclampsia occurred in 1.8% of pregnant people who received the maternal RSV vaccine compared to 1.4% of pregnant people who received a placebo.
The clinical trials identified a small increase in the number of preterm births in vaccinated pregnant people. It is not clear if this is a true safety problem related to RSV vaccine or if this occurred for reasons unrelated to vaccination."
Studies of this RSV vaccine are ongoing, yet the FDA approved it "to reduce the potential risk of preterm birth and complications from RSV disease.
FDA is requiring the manufacturer to do additional studies that will look more closely at the potential risk of preterm births and pregnancy-related high blood pressure issues in mothers, including pre-eclampsia. [Emphasis added.]
I was unable to find CDC infant only RSV mortality statistics for the United States. However, a study, "Infant deaths from respiratory syncytial virus in Lusaka, Zambia from the ZPRIME study: a 3-year, systematic, post-mortem surveillance project," funded by the Bill & Melinda Gates Foundation and published in The Lancet Global Health, found:
"The ZPRIME study ran from Aug 31, 2017, to Aug 31, 2020, except for from April 1 to May 6, 2020, during which data were not collected due to restrictions on human research at this time (linked to COVID-19). We enrolled 2286 deceased infants, representing 79% of total infant deaths in Lusaka. RSV was detected in 162 (7%) of 2286 deceased infants. RSV was detected in 102 (9%) of 1176 community deaths, compared with 10 (4%) of 236 early facility deaths (<48 h from admission) and 36 (5%) of 737 late facility deaths (≥48 h from admission). RSV deaths were concentrated in infants younger than 3 months (116 [72%] of 162 infants), and were clustered in the first half of each year and in the poorest and most densely populated Lusaka townships. RSV caused at least 2·8% (95% CI 1·0–4·6) of all infant deaths and 4·7% (1·3–8·1) of community deaths." [Emphasis added.]
The study's authors' interpretation of this data was:
"RSV was a major seasonal cause of overall infant mortality, particularly among infants younger than 3 months of age. Because most RSV deaths occurred in the community and would have been missed through hospital-based surveillance, the global burden of fatal RSV has probably been underestimated." [Gill, Christopher J., et al. “Infant Deaths from Respiratory Syncytial Virus in Lusaka, Zambia from the ZPRIME Study: A 3-Year, Systematic, Post-Mortem Surveillance Project.” The Lancet Global Health, The Lancet Global Health, Feb. 2022, www.thelancet.com/journals/langlo/article/PIIS2214-109X(21)00518-0/fulltext.]
Another study mentions that delays in care are the rule, not the exception, for Zambian infants, which leads to the logical conclusion that many of the deaths ZPRIME researchers identified as "RSV deaths" may have occurred due to lack of treatment. However, shockingly, the ZPRIME researchers stated:
"The largest concentration of RSV deaths was detected among infants who never reached medical care, and therefore would be systematically missed from previous burden of disease estimates. The fact that most RSV deaths occur outside facility settings suggests that general improvements to health-care delivery are unlikely to substantially reduce RSV deaths in infants. By contrast, preventative strategies, such as vaccines or monoclonal antibodies, could be highly impactful." [Emphasis added.]
Their conclusion that improvements in healthcare delivery would not have substantial positive effects on RSV deaths defies logic since, as you will see, RSV is quite treatable, especially in the absence of other chronic health problems. Vaccines and monoclonal antibodies as the study's proposed solutions make perfect sense given the Bill & Melinda Gates Foundation's cozy relationship with GAVI and Big Pharma. Interestingly, the CDC does not seem to cite the ZPRIME study as proof of RSV-related infant mortality.
So, what symptoms do babies suffer if they contract RSV? Cleveland Clinic states, "Babies younger than 6 months who get RSV may have the following symptoms only:
Fussiness or irritability.
Decreased appetite.
Minimal interest in activities.
Changes in their breathing pattern.
Babies younger than 6 months may require a hospital stay to monitor their breathing and oxygen levels if they get RSV, especially if they have other chronic health issues." [Emphasis added.] It seems quite a stretch to vaccinate pregnant women and their unborn babies, which used to be protected from unnecessary drugs, based on the above symptoms list.
Please read the FDA's press release about Pfizer's new RSV vaccine below.
"Today, the U.S. Food and Drug Administration approved Abrysvo (Respiratory Syncytial Virus Vaccine), the first vaccine approved for use in pregnant individuals to prevent lower respiratory tract disease (LRTD) and severe LRTD caused by respiratory syncytial virus (RSV) in infants from birth through 6 months of age. Abrysvo is approved for use at 32 through 36 weeks gestational age of pregnancy. Abrysvo is administered as a single dose injection into the muscle. The FDA approved Abrysvo in May for the prevention of LRTD caused by RSV in individuals 60 years of age and older.
“RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “This approval provides an option for healthcare providers and pregnant individuals to protect infants from this potentially life-threatening disease.”
RSV is a highly contagious virus that causes respiratory infections in individuals of all age groups. It is the most frequent cause of lower respiratory tract illness in infants worldwide. In most parts of the U.S., RSV circulation is seasonal, typically starting during the fall and peaking in the winter. The virus is especially common in children, and most individuals can be expected to be infected with RSV by the time they reach two years of age. While RSV most often causes cold-like symptoms in infants and young children, it can also lead to serious LRTD such as pneumonia and bronchiolitis (swelling of the small airway passages in the lungs). In infants and children, the risk of RSV-associated LRTD is highest during the first year of life. According to the Centers for Disease Control and Prevention, RSV is the leading cause of infant hospitalization in the U.S.
The safety and effectiveness of Abrysvo for immunization of pregnant individuals to prevent LRTD and severe LRTD caused by RSV in infants from birth through 6 months of age was evaluated in ongoing, randomized, placebo-controlled international clinical studies.
A clinical study evaluated the effectiveness of Abrysvo to prevent LRTD and severe LRTD caused by RSV in infants born to individuals who were vaccinated during pregnancy. Among approximately 3,500 pregnant individuals who received Abrysvo, compared to approximately 3,500 pregnant individuals who received placebo, Abrysvo reduced the risk of severe LRTD by 81.8% within 90 days after birth, and 69.4% within 180 days after birth. In a subgroup of pregnant individuals who were 32 through 36 weeks gestational age, of whom approximately 1,500 received Abrysvo and 1,500 received placebo, Abrysvo reduced the risk of LRTD by 34.7%, and reduced the risk of severe LRTD by 91.1% within 90 days after birth when compared to placebo. Within 180 days after birth, Abrysvo reduced the risk of LRTD by 57.3% and by 76.5% for severe LRTD, when compared to placebo.
The safety of Abrysvo was evaluated in two studies. In one study, approximately 3,600 pregnant individuals received a single dose of Abrysvo and approximately 3,600 pregnant individuals received a placebo. In the second study, approximately 100 pregnant individuals received Abrysvo and approximately 100 pregnant individuals received placebo.
The most commonly reported side effects by pregnant individuals who received Abrysvo were pain at the injection site, headache, muscle pain and nausea.
In addition, although not commonly reported, a dangerous hypertensive disorder, known as pre-eclampsia, occurred in 1.8% of pregnant individuals who received Abrysvo compared to 1.4% of pregnant individuals who received placebo. In the safety studies, low birth weight and jaundice in infants occurred at a higher rate in the pregnant Abrysvo recipients compared to pregnant placebo recipients.
The Prescribing Information for Abrysvo includes a warning to inform that a numerical imbalance in preterm births in Abrysvo recipients (5.7%) occurred compared to those who received placebo (4.7%). The available data are insufficient to establish or exclude a causal relationship between preterm birth and Abrysvo. Specifically, the warning informs healthcare providers that to avoid the potential risk of preterm birth with use of Abrysvo before 32 weeks of gestation, administer Abrysvo as indicated in pregnant individuals at 32 through 36 weeks gestational age. Pregnant individuals who were at increased risk of preterm birth were generally excluded from clinical studies of Abrysvo.
The FDA is requiring the company to conduct postmarketing studies to assess the signal of serious risk of preterm birth and to assess hypertensive disorders of pregnancy, including pre-eclampsia.
The application was granted Priority Review status and Fast Track and Breakthrough Therapy designations.
The FDA granted approval of Abrysvo to Pfizer Inc.
The FDA, an agency within the U.S. Department of Health and Human Services (HHS), protects "the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices." [https://www.fda.gov/about-fda/what-we-do] Is the FDA fulfilling its stated mission by fast tracking a not-fully-studied vaccine to be given to pregnant women to allegedly protect their unborn babies from an already very treatable virus, or is it instead ensuring that the FDA-Big Pharma revolving door keeps spinning?
This article represents the author’s opinion.



This is just another example of fear mongering to create a demand for a vaccine that is unnecessary. Given the hype, it is impossible to make an intelligent risk versus benefit assessment by individuals and their OB/GYN.
Fucking demons. All of them. Fucking demons.